

CCQM-K91.2022
MEASURAND: Acidity function at zero chloride molality, pa0, of an unknown phthalate buffer
SAMPLE: Potassium hydrogen phthalate in deionized water
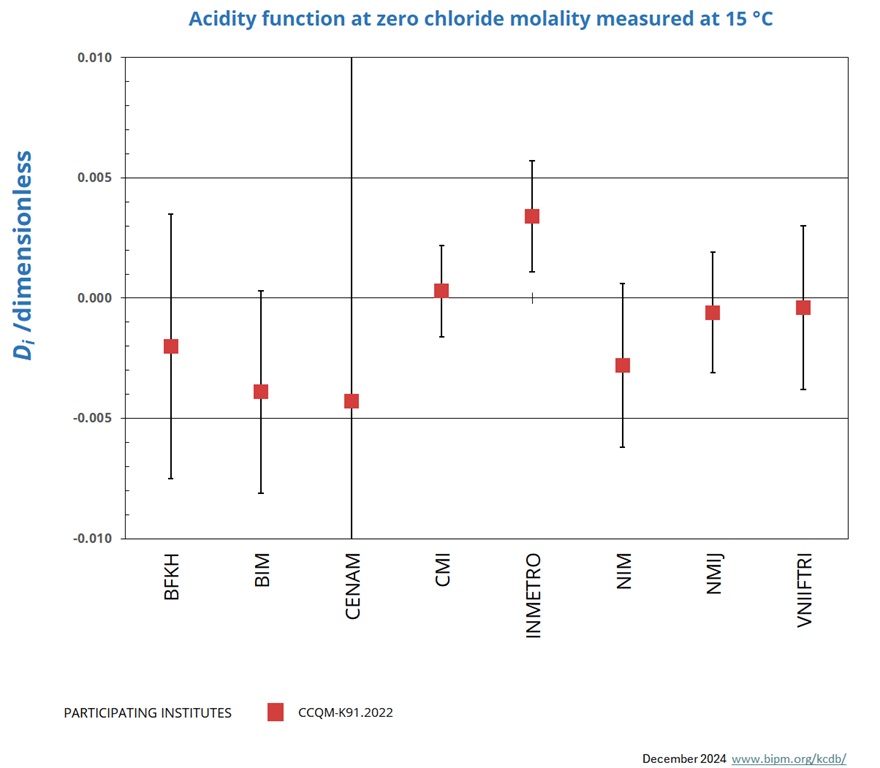
Measurements at 15 °C
NOMINAL VALUE: pH ~ 4.0
Degrees of equivalence represented by Di = (xI - xR) and its expanded uncertainty Ui at a 95 % level of confidence.
Enlarged graph
CCQM-K91.2022
MEASURAND: Acidity function at zero chloride molality, pa0, of an unknown phthalate buffer
SAMPLE: Potassium hydrogen phthalate in deionized water
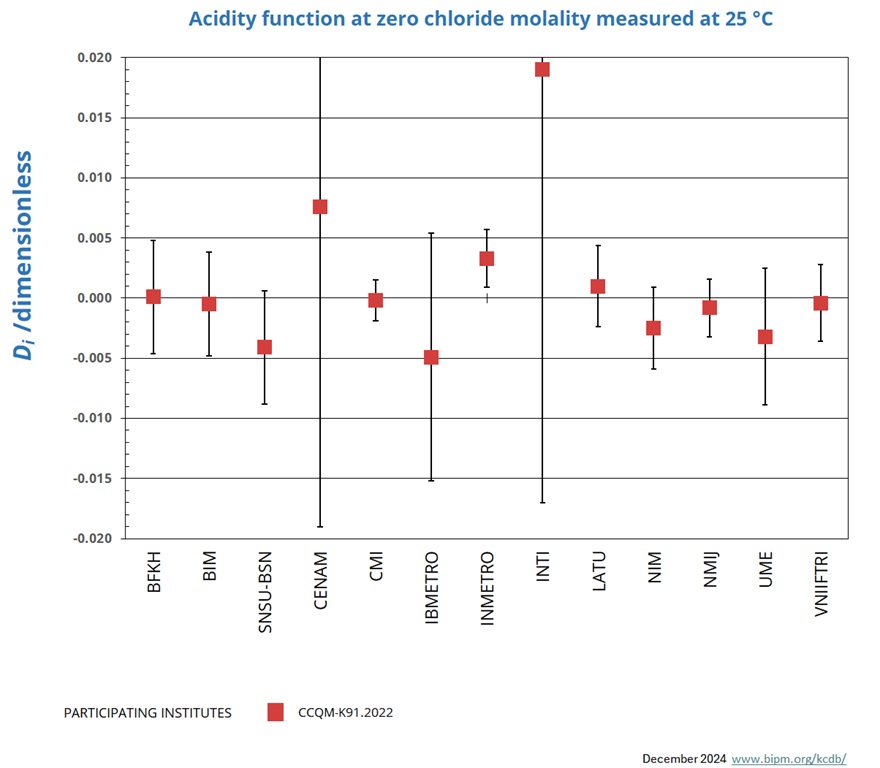
Measurements at 25 °C
NOMINAL VALUE: pH ~ 4.0
Degrees of equivalence represented by Di = (xI - xR) and its expanded uncertainty Ui at a 95 % level of confidence.
Enlarged graph
CCQM-K91.2022
MEASURAND: Acidity function at zero chloride molality, pa0, of an unknown phthalate buffer
SAMPLE: Potassium hydrogen phthalate in deionized water
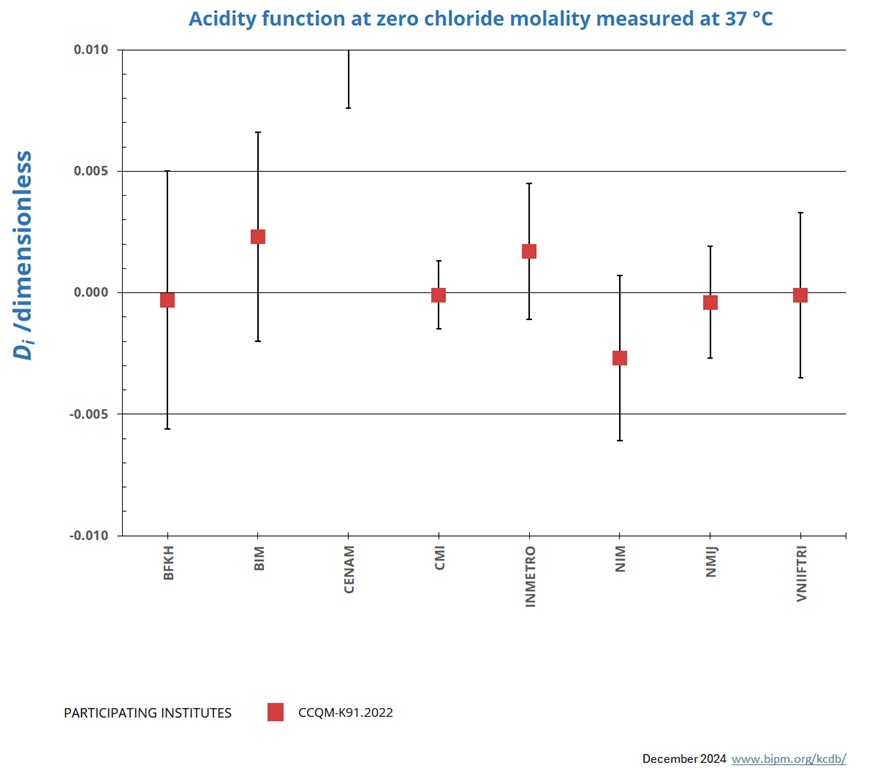
Measurements at 37 °C
NOMINAL VALUE: pH ~ 4.0
Degrees of equivalence represented by Di = (xI - xR) and its expanded uncertainty Ui at a 95 % level of confidence.
Enlarged graph
CCQM-K91.2022
MEASURAND: Acidity function at zero chloride molality, pa0, of an unknown phthalate buffer
SAMPLE: Potassium hydrogen phthalate in deionized water
Measurements at 15 °C
NOMINAL VALUE: pH ~ 4.0
Degrees of equivalence represented by Di = (xI - xR) and its expanded uncertainty Ui at a 95 % level of confidence.
| LABi | Di | Uneg,i | Upos,i | |
|---|---|---|---|---|
dimensionless |
dimensionless |
dimensionless |
||
| BFKH | -0.0020 | 0.0055 | ||
| BIM | -0.0039 | 0.0042 | ||
| CENAM | -0.0043 | 0.0346 | ||
| CMI | 0.0003 | 0.0019 | ||
| INMETRO | 0.0034 | 0.0023 | ||
| NIM | -0.0028 | 0.0034 | ||
| NMIJ | -0.0006 | 0.0025 | ||
| VNIIFTRI | -0.0004 | 0.0034 |
CCQM-K91.2022
MEASURAND: Acidity function at zero chloride molality, pa0, of an unknown phthalate buffer
SAMPLE: Potassium hydrogen phthalate in deionized water
Measurements at 25 °C
NOMINAL VALUE: pH ~ 4.0
Degrees of equivalence represented by Di = (xI - xR) and its expanded uncertainty Ui at a 95 % level of confidence.
| LABi | Di | Uneg,i | Upos,i | |
|---|---|---|---|---|
dimensionless |
dimensionless |
dimensionless |
||
| BFKH | 0.0001 | 0.0047 | ||
| BIM | -0.0005 | 0.0043 | ||
| SNSU-BSN | -0.0041 | 0.0047 | ||
| CENAM | 0.0076 | 0.0266 | ||
| CMI | -0.0002 | 0.0017 | ||
| IBMETRO | -0.0049 | 0.0103 | ||
| INMETRO | 0.0033 | 0.0024 | ||
| INTI | 0.0190 | 0.0360 | ||
| LATU | 0.0010 | 0.0034 | ||
| NIM | -0.0025 | 0.0034 | ||
| NMIJ | -0.0008 | 0.0024 | ||
| UME | -0.0032 | 0.0057 | ||
| VNIIFTRI | -0.0004 | 0.0032 |
CCQM-K91.2022
MEASURAND: Acidity function at zero chloride molality, pa0, of an unknown phthalate buffer
SAMPLE: Potassium hydrogen phthalate in deionized water
Measurements at 37 °C
NOMINAL VALUE: pH ~ 4.0
Degrees of equivalence represented by Di = (xI - xR) and its expanded uncertainty Ui at a 95 % level of confidence.
| LABi | Di | Uneg,i | Upos,i | |
|---|---|---|---|---|
dimensionless |
dimensionless |
dimensionless |
||
| BFKH | -0.0003 | 0.0053 | ||
| BIM | 0.0023 | 0.0043 | ||
| CENAM | 0.0418 | 0.0342 | ||
| CMI | -0.0001 | 0.0014 | ||
| INMETRO | 0.0017 | 0.0028 | ||
| NIM | -0.0027 | 0.0034 | ||
| NMIJ | -0.0004 | 0.0023 | ||
| VNIIFTRI | -0.0001 | 0.0034 |
| Metrology area, Sub-field | Chemistry and Biology, Electrochemistry |
| Description | Acidity function of an unknown phthalate buffer at zero chloride molality |
| Time of measurements | 2022 |
| Status | Approved for equivalence |
| Final Reports of the comparisons | |
| References | |
| Measurand | Acidity function at at zero chloride molality, pa0 (pH) Nominal value of pH ~4.01 at temperature 25 °C |
| Parameters | Temperature: 15 °C, 25 °C and 37 °C, and optional at 5 °C and 50 °C |
| Transfer device | Aqueous phthalate buffer |
| Comparison type | Key Comparison |
| Consultative Committee | CCQM (Consultative Committee for Amount of Substance) |
| Conducted by | CCQM (Consultative Committee for Amount of Substance) |
| Comments | PTB has handed over the coordination to INMETRO |
| Pilot institute |
INMETRO
Instituto Nacional de Metrologia, Qualidade e Tecnologia Brazil |
| Contact person | Fabiano Gonzaga +55-21-26799134 |
| Pilot laboratory | |
|---|---|
| INMETRO |
Instituto Nacional de Metrologia, Qualidade e Tecnologia, Brazil, SIM |
| BFKH |
Government Office of the Capital City Budapest, Hungary, EURAMET |
| BIM |
Bulgarian Institute of Metrology, Bulgaria, EURAMET |
| CENAM |
Centro Nacional de Metrologia, Mexico, SIM |
| CMI |
Czech Metrology Institute, Czechia, EURAMET |
| IBMETRO |
Instituto Boliviano de Metrología, Bolivia, SIM |
| INTI |
Instituto Nacional de Tecnologia Industrial, Argentina, SIM |
| LATU |
Laboratorio Tecnologico del Uruguay, Uruguay, SIM |
| NIM |
National Institute of Metrology, China, APMP |
| NMIJ AIST |
National Metrology Institute of Japan, Japan, APMP |
| SNSU-BSN |
National Measurement Standard - National Standardization Agency of Indonesia, Indonesia, APMP |
| UME |
TÜBITAK Ulusal Metroloji Enstitüsü, Türkiye, EURAMET |
| VNIIFTRI |
Institute of Physical Technical and Radiotechnical Measurements, Rosstandart, Russian Federation, COOMET |
This page proposes print-out on A4 paper (portrait) of the comparison details (best printed out using a black and white printer).
Please, select items to be printed out, then click on "OK" :
CCQM-K91.2022
MEASURAND: Acidity function at zero chloride molality, pa0, of an unknown phthalate buffer
SAMPLE: Potassium hydrogen phthalate in deionized water
Measurements at 15 °C
NOMINAL VALUE: pH ~ 4.0
The key comparison reference value xR and its associated standard uncertainty uR are computed from the weighted mean uncorrected for observed dispersion according to equation 7 to 9 of the CCQM-K91.2022 Final report, from results obtained using primary method CENAM excluded.
xR = 4.0873 uR = 0.0006
The degree of equivalence of laboratory i relative to the key comparison reference value xR is given by a pair of terms:
Di = (xi - xR) and its expanded uncertainty Ui at a 95 % level of confidence where Ui = 2 (ui 2 - uR2)1/2 for resuts used in calculation of xR and
Ui = 2(ui 2 + uR2)1/2 for results not used in the calculation of xR.
CCQM-K91.2022
MEASURAND: Acidity function at zero chloride molality, pa0, of an unknown phthalate buffer
SAMPLE: Potassium hydrogen phthalate in deionized water
Measurements at 25 °C
NOMINAL VALUE: pH ~ 4.0
The key comparison reference value xR and its associated standard uncertainty uR are computed from the weighted mean uncorrected for observed dispersion according to equation 7 to 9 of the CCQM-K91.2022 Final report, from results obtained using primary method CENAM excluded.
xR = 4.0956 uR = 0.0005
The degree of equivalence of laboratory i relative to the key comparison reference value xR is given by a pair of terms:
Di = (xi - xR) and its expanded uncertainty Ui at a 95 % level of confidence where Ui = 2 (ui 2 - uR2)1/2 for resuts used in calculation of xR and
Ui = 2(ui 2 + uR2)1/2 for results not used in the calculation of xR.
CCQM-K91.2022
MEASURAND: Acidity function at zero chloride molality, pa0, of an unknown phthalate buffer
SAMPLE: Potassium hydrogen phthalate in deionized water
Measurements at 37 °C
NOMINAL VALUE: pH ~ 4.0
The key comparison reference value xR and its associated standard uncertainty uR are computed from the weighted mean uncorrected for observed dispersion according to equation 7 to 9 of the CCQM-K91.2022 Final report, from results obtained using primary method CENAM excluded.
xR = 4.1159 uR = 0.0006
The degree of equivalence of laboratory i relative to the key comparison reference value xR is given by a pair of terms:
Di = (xi - xR) and its expanded uncertainty Ui at a 95 % level of confidence where Ui = 2 (ui 2 - uR2)1/2 for resuts used in calculation of xR and
Ui = 2(ui 2 + uR2)1/2 for results not used in the calculation of xR.
CCQM-K91.2022
MEASURAND: Acidity function at zero chloride molality, pa0, of an unknown phthalate buffer
SAMPLE: Potassium hydrogen phthalate in deionized water
Measurements at 15 °C
NOMINAL VALUE: pH ~ 4.0
xi measured mass fraction reported by laboratory i, Labi
ui combined standard uncertainty of xi
| Labi | xi | ui | |
| dimensionless | dimensionless | ||
| BFKH | 4.0853 | 0.0028 | |
| BIM | 4.0834 | 0.0022 | |
| CENAM | 4.0830 | 0.0173 | |
| CMI | 4.0876 | 0.0011 | |
| INMETRO | 4.0907 | 0.0013 | |
| NIM | 4.0845 | 0.0018 | |
| NMIJ | 4.0867 | 0.0014 | |
| VNIIFTRI | 4.0869 | 0.0018 |
CCQM-K91.2022
MEASURAND: Acidity function at zero chloride molality, pa0, of an unknown phthalate buffer
SAMPLE: Potassium hydrogen phthalate in deionized water
Measurements at 25 °C
NOMINAL VALUE: pH ~ 4.0
xi measured mass fraction reported by laboratory i, Labi
ui combined standard uncertainty of xi
| Labi | xi | ui | |
| dimensionless | dimensionless | ||
| BFKH | 4.0957 | 0.0024 | |
| BIM | 4.0951 | 0.0022 | |
| SNSU-BSN | 4.0915 | 0.0023 | |
| CENAM | 4.1032 | 0.0133 | |
| CMI | 4.0954 | 0.0010 | |
| IBMETRO | 4.0907 | 0.0051 | |
| INMETRO | 4.0989 | 0.0013 | |
| INTI | 4.1146 | 0.0180 | |
| LATU | 4.0966 | 0.0016 | |
| NIM | 4.0931 | 0.0018 | |
| NMIJ | 4.0948 | 0.0013 | |
| UME | 4.0924 | 0.0029 | |
| VNIIFTRI | 4.0952 | 0.0017 |
CCQM-K91.2022
MEASURAND: Acidity function at zero chloride molality, pa0, of an unknown phthalate buffer
SAMPLE: Potassium hydrogen phthalate in deionized water
Measurements at 37 °C
NOMINAL VALUE: pH ~ 4.0
xi measured mass fraction reported by laboratory i, Labi
ui combined standard uncertainty of xi
| Labi | xi | ui | |
| dimensionless | dimensionless | ||
| BFKH | 4.1156 | 0.0027 | |
| BIM | 4.1182 | 0.0022 | |
| CENAM | 4.1577 | 0.0171 | |
| CMI | 4.1158 | 0.0009 | |
| INMETRO | 4.1176 | 0.0015 | |
| NIM | 4.1132 | 0.0018 | |
| NMIJ | 4.1155 | 0.0013 | |
| VNIIFTRI | 4.1158 | 0.0018 |